Unit 4: Atomic Structure
Reading
BJU Chemistry: Ch. 4 "Atomic Structure"
AP Classroom: Unit 1 "Atomic Structure and Properties" (continued)
AP Princeton Review: Unit 1 (continued)
Topics
Labs
BJU Chemistry: Ch. 4 "Atomic Structure"
AP Classroom: Unit 1 "Atomic Structure and Properties" (continued)
AP Princeton Review: Unit 1 (continued)
Topics
- Structure of the atom
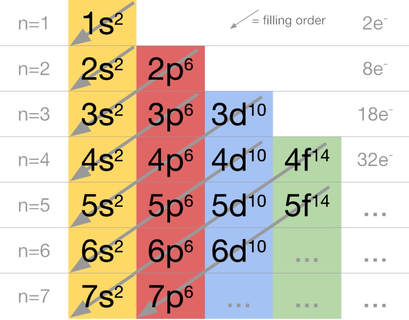
- Electron configurations
- Atomic number and atomic mass
- Isotopes
- Ions
Labs
- Oxygen lab: Prepare and test oxygen gas
- Build-an-Atom weblab
| 3._ch._4_atomic_structure_homework_tips_notes_2023.docx |
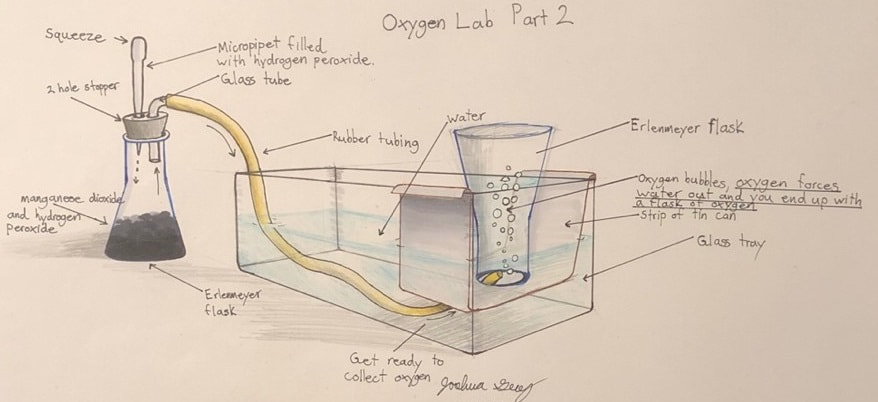
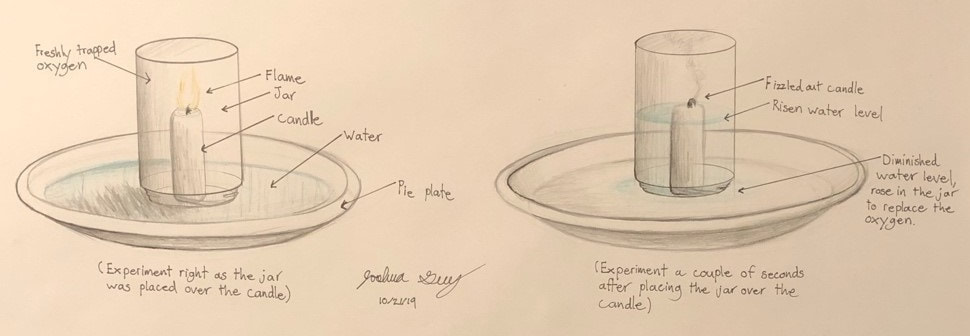
Oxygen lab
We will make and test oxygen gas. This is a classic lab, making and collecting an atmospheric gas, and testing it under different conditions. Remember to bring the lab handout!
We will make and test oxygen gas. This is a classic lab, making and collecting an atmospheric gas, and testing it under different conditions. Remember to bring the lab handout!
| o2_lab_handout.pdf |
Homework
The chapter homework, and various other projects are posted in Canvas.
The chapter homework, and various other projects are posted in Canvas.
Build-an-Atom Weblab
| 3._build-an-atom_weblab_2023.doc |
Optional: Elements of the Human Body research assignment
Problem statement: The four most abundant elements by mass in the human body are oxygen, carbon, hydrogen, and nitrogen. These four elements make up about 96% of the human body. The next four most abundant elements are calcium, phosphorus, magnesium, and potassium.
Problem statement: The four most abundant elements by mass in the human body are oxygen, carbon, hydrogen, and nitrogen. These four elements make up about 96% of the human body. The next four most abundant elements are calcium, phosphorus, magnesium, and potassium.
- Write the ground-state electron configurations for these eight most abundant elements in the human body.
- Then choose any one of the eight; research and explain how the body obtains that element, and what function that element performs in the body (what does it do, chemically speaking?). Include pictures and diagrams, chemical equations, and any other information which helps explain its source & function in the body. This section should be 1-1/2 to 2 pages in length including diagrams.
Videos of standing waves
It's difficult to picture electrons as 'waves' which must occupy discrete 'orbitals' or energy-levels. If you think this is confusing, you're not alone!
The vibrating "Chlodni" plates (watch videos below) might help you visualize what standing waves look like - at least in 2 dimensions anyway. As the vibration frequency is increased in the videos (representing more and more energy input), the salt crystals arrange themselves at the wave "nodes". The steel plate vibrates as a wave, and the salt can only exist at the nodes, where there is no vibration.
In the same way, electrons can occupy only "discrete" orbitals - or energy levels - around an atom. They can't just sit anywhere they want; they must occupy discrete energy levels (orbitals or shells) which are numbered 1s, 2s, 2p, etc.
When you add energy (light of a certain wavelength, for example) to an atom, the electrons can jump up to a higher orbital. Conversely, when electrons jump down a level, light energy is emitted from the atom.
It's difficult to picture electrons as 'waves' which must occupy discrete 'orbitals' or energy-levels. If you think this is confusing, you're not alone!
The vibrating "Chlodni" plates (watch videos below) might help you visualize what standing waves look like - at least in 2 dimensions anyway. As the vibration frequency is increased in the videos (representing more and more energy input), the salt crystals arrange themselves at the wave "nodes". The steel plate vibrates as a wave, and the salt can only exist at the nodes, where there is no vibration.
In the same way, electrons can occupy only "discrete" orbitals - or energy levels - around an atom. They can't just sit anywhere they want; they must occupy discrete energy levels (orbitals or shells) which are numbered 1s, 2s, 2p, etc.
When you add energy (light of a certain wavelength, for example) to an atom, the electrons can jump up to a higher orbital. Conversely, when electrons jump down a level, light energy is emitted from the atom.