Unit 16: Acids & Bases
Reading
BJU Chemistry: Ch. 16 "Acids, Bases, and Salts"
AP Classroom: Unit 8 "Acids and Bases"
AP Princeton Review: Unit 8
Topics
Labs
BJU Chemistry: Ch. 16 "Acids, Bases, and Salts"
AP Classroom: Unit 8 "Acids and Bases"
AP Princeton Review: Unit 8
Topics
- Properties of acids and bases
- The pH scale
- The properties of buffers
- Acid-base titration
Labs
- Acid-Base Titration lab
- pH Scale weblab
- Acids & Bases weblab
| ch._16_acids_&_bases_homework_help.docx |
| 16._acids_and_bases_class_notes1.docx |
For an excellent (brief) overview of Acids & Bases, click here https://chem.libretexts.org/..../Overview_of_Acids_and_Bases
Acid-Base Titration lab
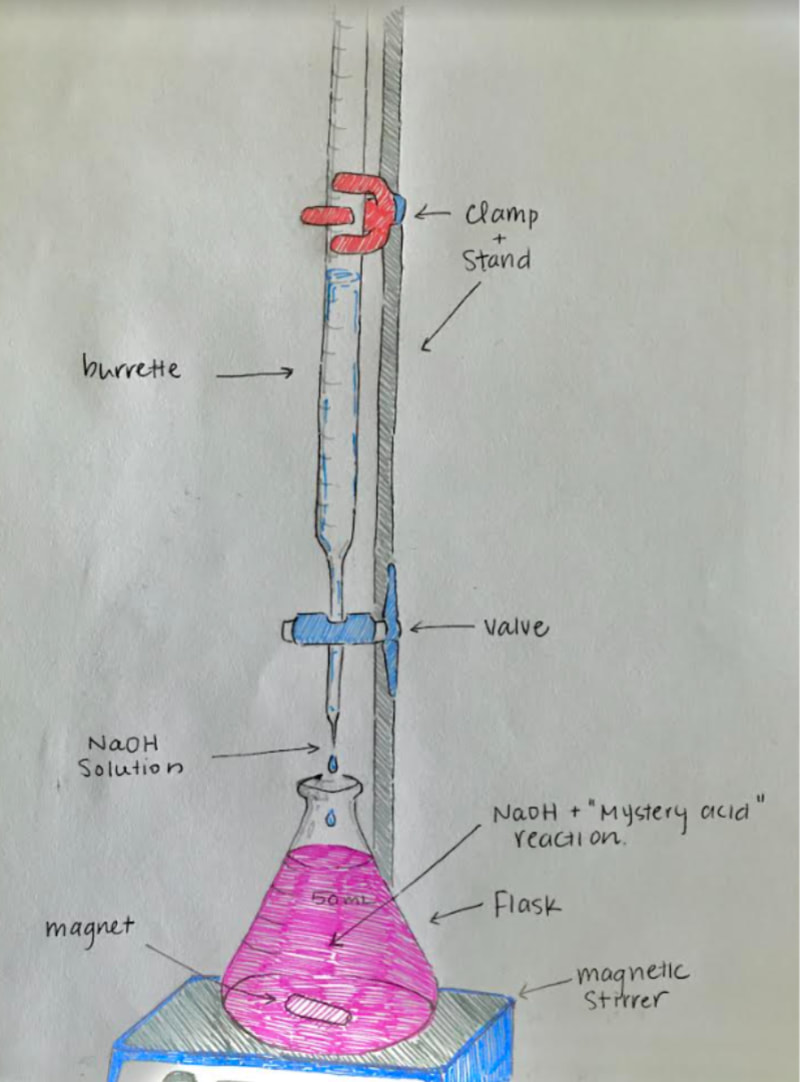
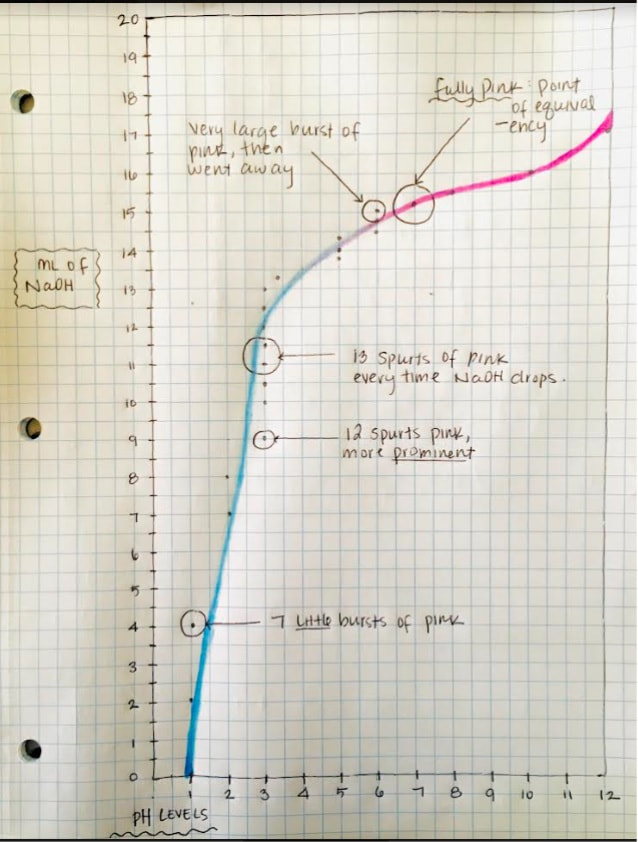
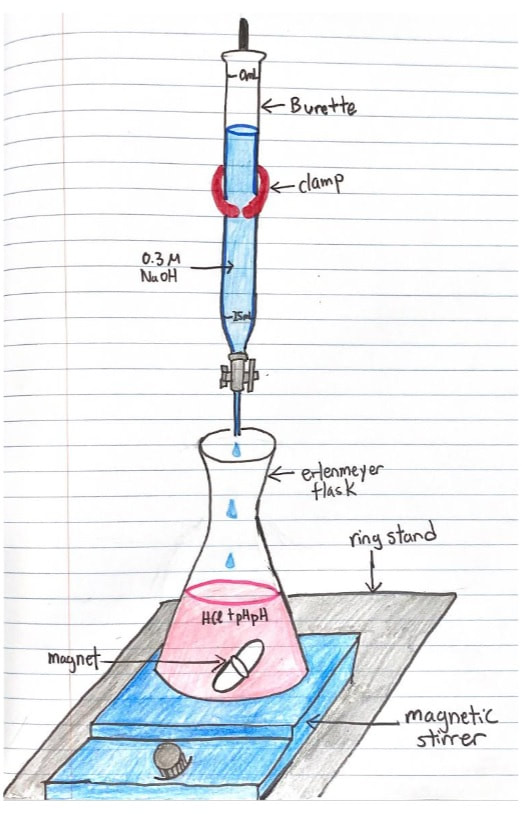
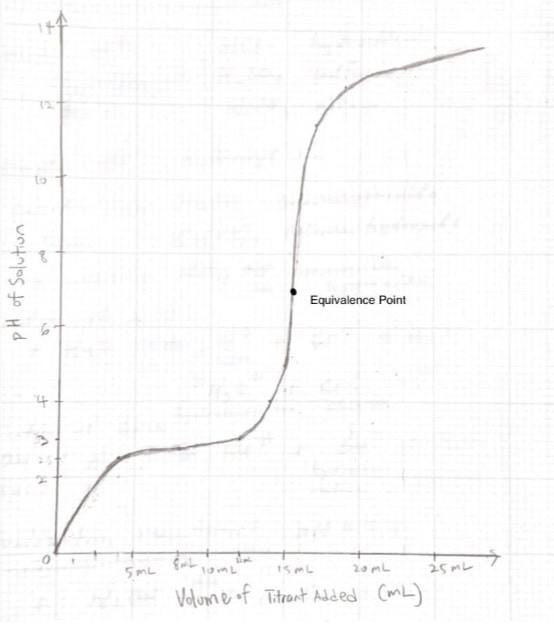
This is the classic acid-base lab exercise. We will use glass burettes (handle them carefully!), mag stirrers, hydrochloric acid, and sodium hydroxide. The pH indicator will be phenolphthalien. The titration process IS COVERED IN CHAPTER 16 OF YOUR BOOK. Read this section before the lab.
This is the classic acid-base lab exercise. We will use glass burettes (handle them carefully!), mag stirrers, hydrochloric acid, and sodium hydroxide. The pH indicator will be phenolphthalien. The titration process IS COVERED IN CHAPTER 16 OF YOUR BOOK. Read this section before the lab.
| acid-base_titration_handout.docx |
Weblabs
| 0._acids_and_bases_weblab_2024__ |
| 16._ph_scale_weblab.docx |
Acids & Bases problems
See Canvas
See Canvas