Unit 2: Properties of Matter
Reading:
BJU Chemistry: Ch. 2 "Matter"
AP Classroom: Unit 1 "Atomic Structure and Properties"
AP Princeton Review: Unit 1
Topics
Auto Crash Bag research assignment
Instructions below
Lab
"Elements, Compounds, and Mixtures" lab and "Sulfur Compounds" lab. Instructions below. Remember to bring both lab handouts!
Virtual labs that also illustrate the topic
BJU Chemistry: Ch. 2 "Matter"
AP Classroom: Unit 1 "Atomic Structure and Properties"
AP Princeton Review: Unit 1
Topics
- Elements, compounds, and mixtures
- Physical and chemical properties
- States of matter
- Energy conversions
- Temperature conversions
Auto Crash Bag research assignment
Instructions below
Lab
"Elements, Compounds, and Mixtures" lab and "Sulfur Compounds" lab. Instructions below. Remember to bring both lab handouts!
Virtual labs that also illustrate the topic
- PhET Energy Forms and Changes weblab
- PhET States of Matter weblab
| 2._matter_handout__with_4_illustrations |
Homework handouts
Other homework will be posted in Canvas.
AP students will also have some problems posted in AP Classroom.
Other homework will be posted in Canvas.
AP students will also have some problems posted in AP Classroom.
Below: Lecture video Ch.2, 9/12/2023
| 2._matter_homework_questions__rev_2023_.docx |
Below: The famous "Elephant Toothpaste" demonstration. It uses hydrogen peroxide (H2O2), liquid soap, and a catalyst such as manganese dioxide (MnO2) or potassium iodide (KI). The catalyst causes the H2O2 to rapidly break down, producing copious amounts of foaming oxygen gas. If you remind me, we can quickly set this up and demonstrate it (on a smaller scale).
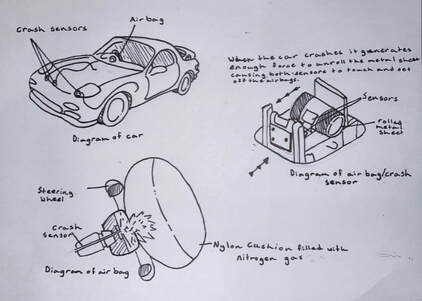
Auto Crash Bag research assignment
A car air bag explodes in just 1/100th of a second when you collide.
Research and submit a 1-1/2 to 2 page report in Canvas. Your report will be graded on the following:
Make sure your report includes:
A car air bag explodes in just 1/100th of a second when you collide.
Research and submit a 1-1/2 to 2 page report in Canvas. Your report will be graded on the following:
- How does this happen, and what is going on chemically?
- Include sketches/diagrams of the mechanical parts,
- the chemical reactions taking place,
- sketches or models of the key molecules involved,
- and any relevant pictures.
Make sure your report includes:
- Sketches/diagrams
- Chemical reactions
| auto_crash_bag_student_exemplar_papers.pdf |
Elements, Compounds, and Mixtures lab and Sulfur Compounds lab
- We will start off by observing and testing the properties of two common elements, iron (Fe) and sulfur (S). This includes dissolving the iron in acid and trying to dissolve (unsuccessfully) the pure sulfur in various things.
- Then we will carry out a reaction which combines the Fe and S to form FeS, a completely new compound with a different appearance and properties.
- After that, we will react a small amount of the FeS with hydrochloric acid (HCl) to form hydrogen sulfide gas (H2S) which has quite different (and noticeable!) properties.
- Finally, we will do some experiments with the element sulfur.
- All of this serves as an excellent introduction to elements and compounds, and setting up chemical reactions.
| _elements_compounds_mixtures_lab_handout_.pdf |
| sulfur_compounds_lab_handout.pdf |