Unit 11: Solids and Liquids
Reading
BJU Chemistry: Ch. 11 "Solids and Liquids"
AP Classroom: Unit 3 "Intermolecular Forces and Properties" (continued)
AP Princeton Review: Unit 3 (continued)
Topics
Possible labs (we'll decide when we get here)
BJU Chemistry: Ch. 11 "Solids and Liquids"
AP Classroom: Unit 3 "Intermolecular Forces and Properties" (continued)
AP Princeton Review: Unit 3 (continued)
Topics
- Intermolecular forces: Hydrogen bonds, dipole-dipole forces, dispersion (London) forces.
- Properties of solids and liquids
- Separation processes: distillation, crystallization
- Measuring melting points, freezing points
Possible labs (we'll decide when we get here)
- Boron Compounds lab, together with flame testing of metals to observe emission spectra.
- Distillation lab
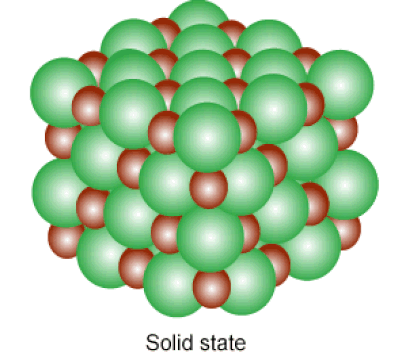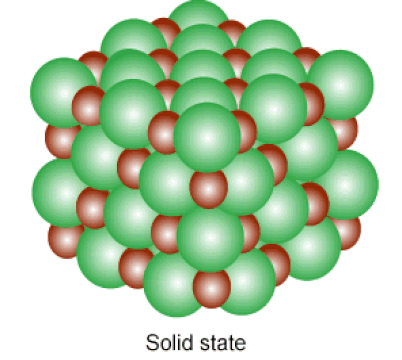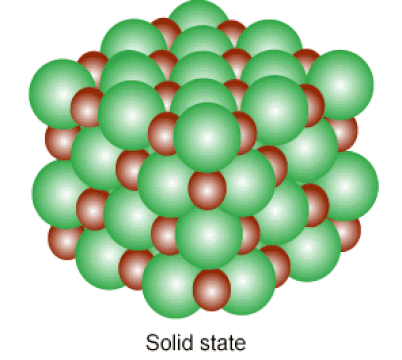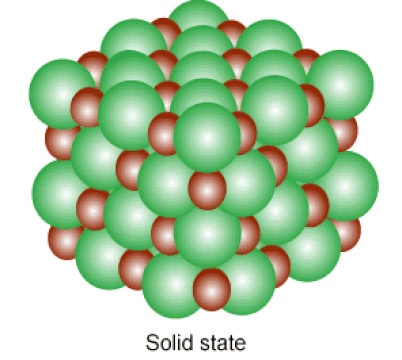
Below: Solids and liquids are held together by INTERmolecular forces. The atoms in a solid vibrate in place, while the atoms in a liquid can slide past one another.
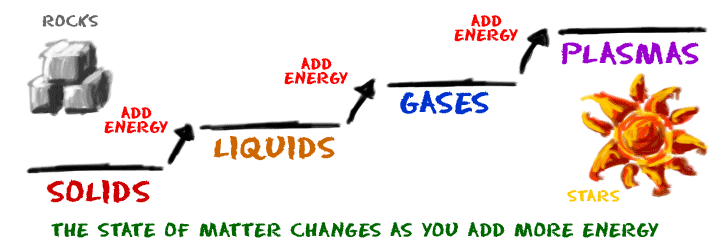
Below: The four states of matter. As you add heat to a solid, the atoms increasingly vibrate and start sliding past one another, at which point you have a liquid. As even more heat is added, the atoms begin to leave entirely and take on the gaseous phase. Under intense heat, even the subatomic particles break apart and you have a plasma.
Lecture overview
Up to this point we have been discussing INTRAmolecular forces, the forces which BOND ATOMS together. These fall into three broad categories: 1) Ionic bonds, 2) Covalent bonds, and 3) Polar-covalent bonds. We also discussed Metallic bonds, as well.
Now we are switching gears and talking about INTERmolecular forces, the forces which hold molecules together to form LIQUIDS and SOLIDS.
These INTERmolecular forces come in three types:
All three of these intermolecular forces are termed "Van der Waal" forces. This can sometimes be a source of confusion, so now you have been warned.
Intermolecular forces are WEAK forces: A hydrogen bond represents only about 20 KJ/mol, and Dispersion forces only represent <5 KJ/mol in strength - whereas a typical Covalent bond is around 400-500 KJ/mol. If intermolecular forces were much stronger we wouldn't have any liquids or gases in nature; everything would just be a SOLID, and life wouldn't exist! Is this evidence of fine-tuning?
Up to this point we have been discussing INTRAmolecular forces, the forces which BOND ATOMS together. These fall into three broad categories: 1) Ionic bonds, 2) Covalent bonds, and 3) Polar-covalent bonds. We also discussed Metallic bonds, as well.
Now we are switching gears and talking about INTERmolecular forces, the forces which hold molecules together to form LIQUIDS and SOLIDS.
These INTERmolecular forces come in three types:
- Dipole-dipole forces, the electrostatic attraction between the (+) and (-) ends of polar molecules
- Hydrogen bonds, the electrostatic attraction between a Hydrogen on one compound, and either Oxygen, Nitrogen, or Fluorine on an adjacent compound
- Dispersion forces (London forces), the fairly-weak electrostatic attraction between non-polar molecules which arises from an unequal distribution of their respective electron-clouds at any given instant.
All three of these intermolecular forces are termed "Van der Waal" forces. This can sometimes be a source of confusion, so now you have been warned.
Intermolecular forces are WEAK forces: A hydrogen bond represents only about 20 KJ/mol, and Dispersion forces only represent <5 KJ/mol in strength - whereas a typical Covalent bond is around 400-500 KJ/mol. If intermolecular forces were much stronger we wouldn't have any liquids or gases in nature; everything would just be a SOLID, and life wouldn't exist! Is this evidence of fine-tuning?
| chemical_bonding_chart_-_compares_common_bond_strengths.pdf |
Boron Compounds lab
- Preparation of some compounds with interesting properties based on the element 'Boron'
- Flame tests on various metals to observe emission spectra.
| boron_compounds_lab_handout.pdf |
| flame_test_chart_metal_ions.png |
Homework
Handout(s) below. Additional homework is hosted in Canvas.
Handout(s) below. Additional homework is hosted in Canvas.
| 11._solids___liquids_questions_bju_ch._11.docx |