Unit 9: Chemical Calculations
Reading
BJU Chemistry: Ch. 9 "Chemical Calculations"
AP Classroom: Unit 4 "Chemical Reactions" (continued)
AP Princeton Review: Unit 4 (continued)
Topics
Labs
BJU Chemistry: Ch. 9 "Chemical Calculations"
AP Classroom: Unit 4 "Chemical Reactions" (continued)
AP Princeton Review: Unit 4 (continued)
Topics
- Stoichiometry
- Calculations involving chemical reactions
- Limiting reactant and percent yield
Labs
- Electrolysis of Water
| 9._chemical_calculations_lecture_part_1__section_9a_.docx |
| 9._chemical_calculations_lecture_part_2__section_9b_.docx |
Below: the famous "sugar snake" experiment, reacting sulfuric acid with sugar. This is an example of an 'oxidation' reaction, wherein the acid oxidizes the carbon contained in the sugar. If someone reminds me, we can perhaps set this up after our regular lab session.
Why do we need "calculations"? Consider this scenario....
After 6 months of patiently experimenting in your amazing garage laboratory, you create a new compound which we shall call "C", by way of the following reaction: 2A + 3B = C. This is super-exciting because "C" turns out to be a highly sought-after compound which you can sell to pharmaceutical companies for $100 per gram!... whereas "A" and "B" are commonly-used chemicals which only cost a few dollars per kilogram! Realizing the enormous profits to be made, you set out to calculate the amounts of A and B you need in order to produce a set amount of C. In order to do this, you need to employ what we call stoichiometry (stoichometry = "element" + "measure").
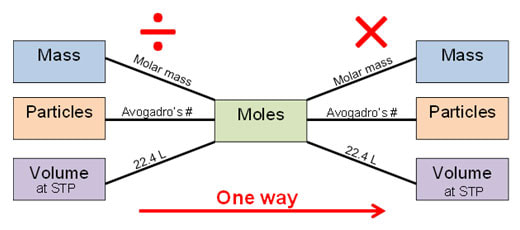
To do these types of chemistry calculations, we use what is called "mole bridge". This means we convert everything to moles first, before crossing over from reactants to products in the chemical equation. Then, we can safely convert back to the number of grams or liters, depending on what we're after.
After 6 months of patiently experimenting in your amazing garage laboratory, you create a new compound which we shall call "C", by way of the following reaction: 2A + 3B = C. This is super-exciting because "C" turns out to be a highly sought-after compound which you can sell to pharmaceutical companies for $100 per gram!... whereas "A" and "B" are commonly-used chemicals which only cost a few dollars per kilogram! Realizing the enormous profits to be made, you set out to calculate the amounts of A and B you need in order to produce a set amount of C. In order to do this, you need to employ what we call stoichiometry (stoichometry = "element" + "measure").
To do these types of chemistry calculations, we use what is called "mole bridge". This means we convert everything to moles first, before crossing over from reactants to products in the chemical equation. Then, we can safely convert back to the number of grams or liters, depending on what we're after.
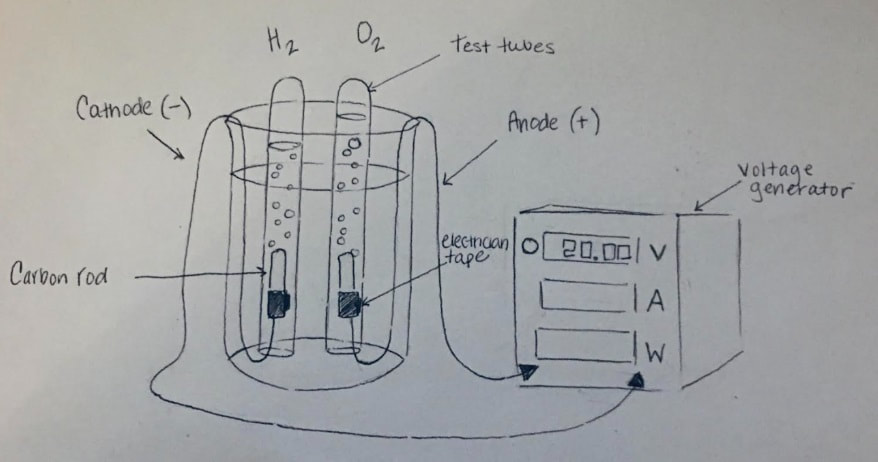
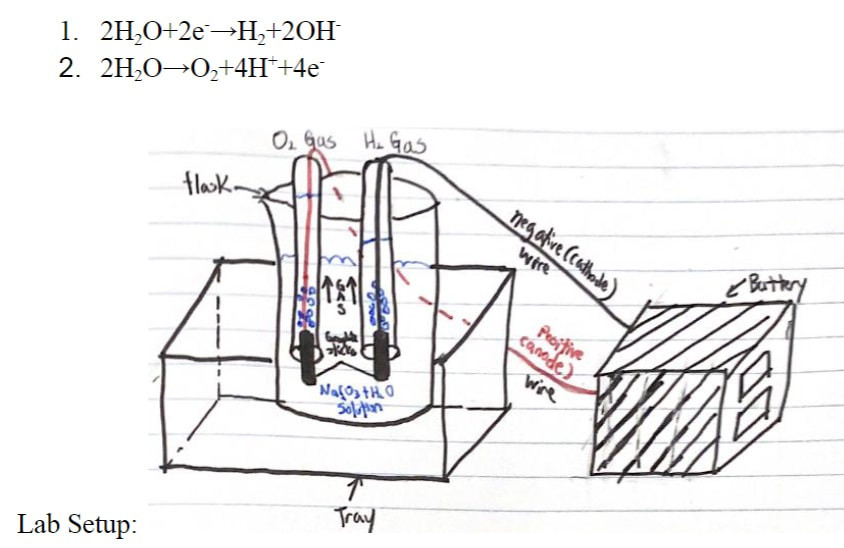
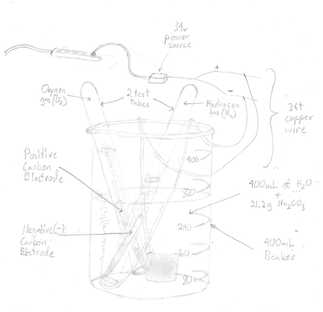
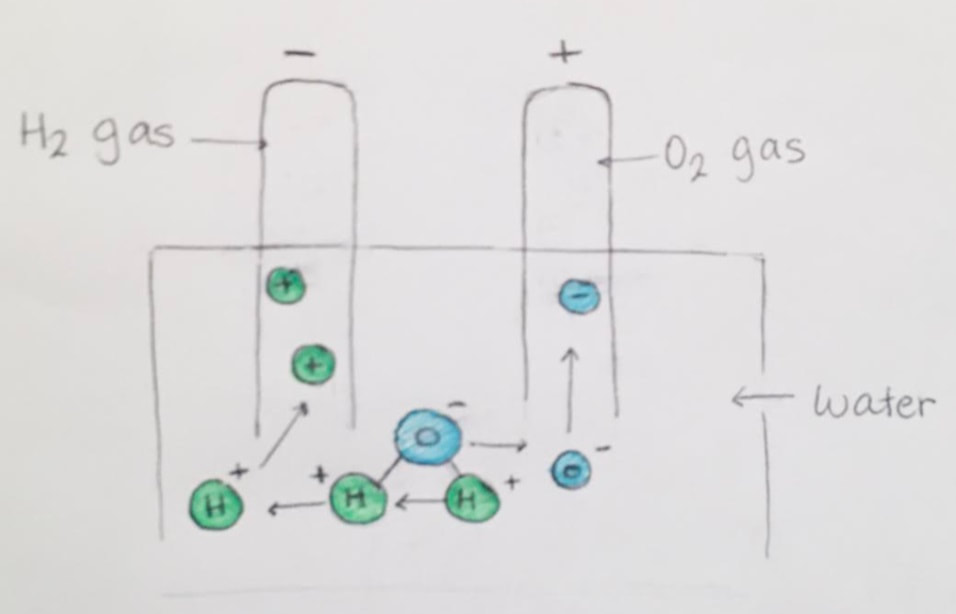
Electrolysis of Water lab
"Electro" (involving electricity) + "Lysis" (to split apart) is where we use DC voltage to carry out a "decomposition" reaction.
We will set up two, very common, electrolytic processes.... and observe what happens.
"Electro" (involving electricity) + "Lysis" (to split apart) is where we use DC voltage to carry out a "decomposition" reaction.
- Electrolysis is very important in modern industrial processes.
- You can do a lot of interesting and profitable chemistry by inserting electrodes into compounds and running electricity through the compound at around 5-20 volts.
We will set up two, very common, electrolytic processes.... and observe what happens.
- Electrolysis of Water into hydrogen and oxygen gases
- Electrolytic production of sodium hypochlorite bleach from sodium chloride
| electrolysis_of_water_lab.pdf |
| _know_your_ions_homework_questions_rev._2020.docx |