Unit 8: Chemical Composition & Reactions
Reading
BJU Chemistry: Ch. 8 "Chemical Composition and Reactions"
AP Classroom: Unit 4 "Chemical Reactions"
AP Princeton Review: Unit 4
Topics
Labs
Virtual labs which pertain to the topics covered
BJU Chemistry: Ch. 8 "Chemical Composition and Reactions"
AP Classroom: Unit 4 "Chemical Reactions"
AP Princeton Review: Unit 4
Topics
- Types of chemical reactions
- Oxidation numbers
- Naming compounds
- Writing and balancing chemical equations
Labs
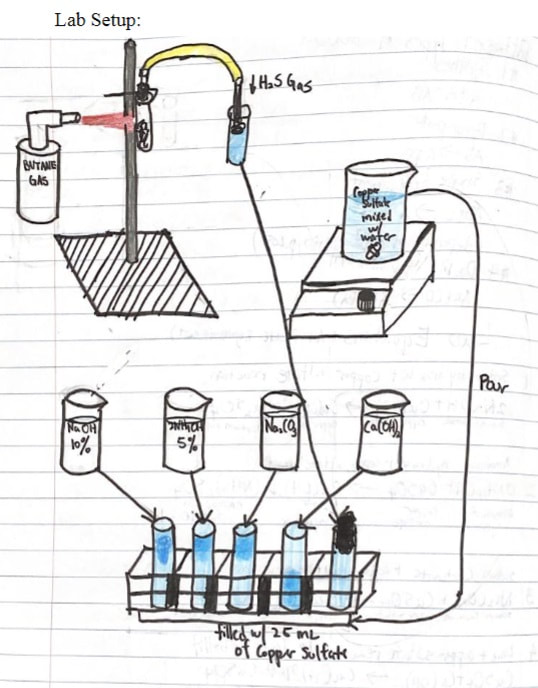
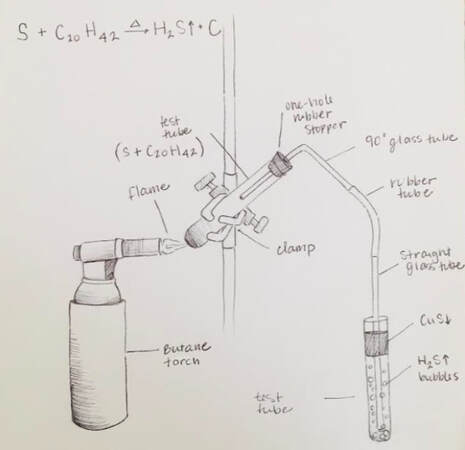
- Copper Compounds lab. Make strikingly-colored compounds which illustrate virtually every topic in this chapter.
- Oil Spill lab: Create our own oil spill and test various chemical methods of cleanup
- Optional lab: Separation of a Ternary Mixture
Virtual labs which pertain to the topics covered
- "Balancing Chemical Equations" PhET weblab
- "Reactants, Products, and Leftovers" PhET weblab (both these weblabs are excellent)
| 7._reactions_oxidation_numbers_lecture_notes.docx |
| 7._chemical_reactions_homework_2023.docx |
Copper Compounds lab
This is a classic experiment done in chemistry labs since the beginning of chemistry labs. Remember to bring the lab handout!
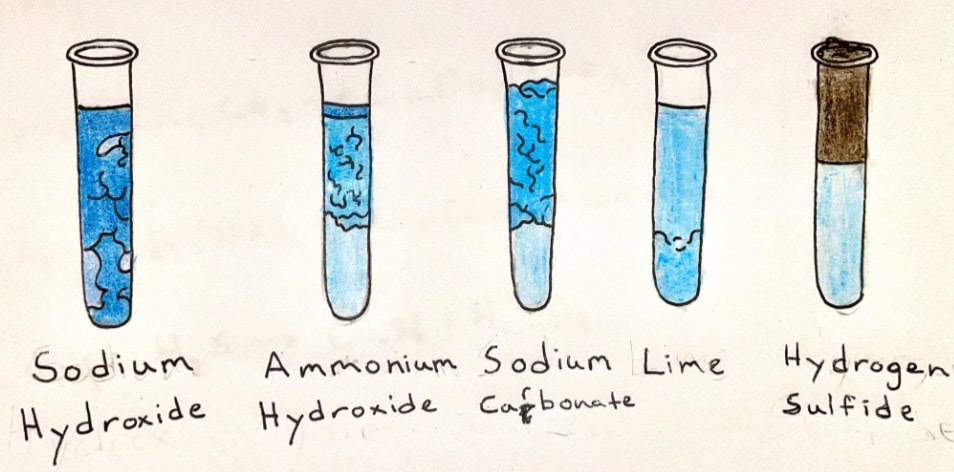
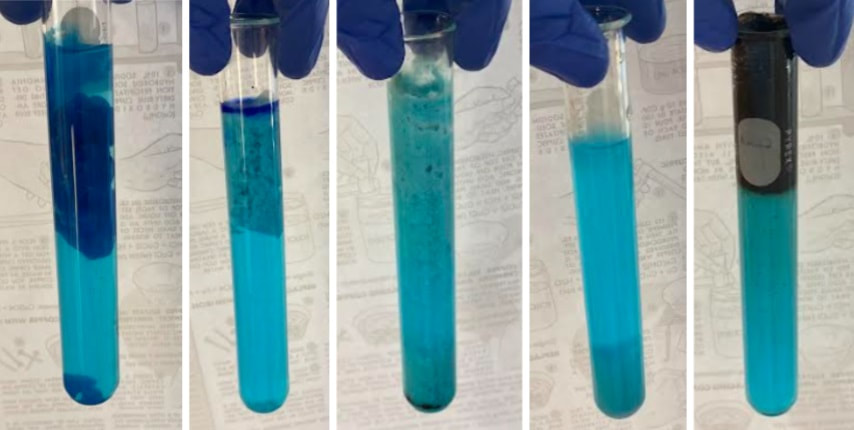
Copper makes two kinds of salts:
This is a classic experiment done in chemistry labs since the beginning of chemistry labs. Remember to bring the lab handout!
Copper makes two kinds of salts:
- Cuprous salts (such as cuprous chloride, CuCl) are colorless.
- Cupric salts (such as cupric sulfate, CuSO4) have a range of bright blue colors.
- We can easily make several copper compounds which demonstrate synthesis, replacement, and decomposition reactions. The end results are usually brilliant blue, or greenish-blue, compounds that are pretty cool to look at.
| copper_compounds_lab_handout_with_equations_2pp.pdf |
| copper_compounds_lab_report_student_exemplar.docx |
| copper_compounds_lab_report_STUDENT EXEMPLAR.docx |
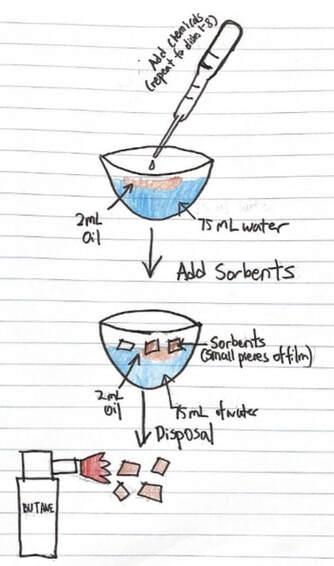
Oil Spill lab
Create an 'oil spill' and test various chemical methods of cleanup, including dispersants, detergents, acids, bases, solvents, direct burning, and absorbent 'blankets'
Create an 'oil spill' and test various chemical methods of cleanup, including dispersants, detergents, acids, bases, solvents, direct burning, and absorbent 'blankets'
Advanced lab: Separation of a Ternary Mixture
| separating_a_ternary_mixture_instructions.docx |
| separating_a_ternary_mixture_student_worksheet.docx |