Unit 7: Molecular Shape
Reading
BJU Chemistry book: Ch. 7 "Bond Theories and Molecular Geometry"
AP Classroom: Unit 2 "Molecular and Ionic Compound Structure and Properties" (continued)
AP Princeton Review: Unit 2 (continued)
Topics
Labs
Oil Spill Cleanup research assignment
Instructions below
BJU Chemistry book: Ch. 7 "Bond Theories and Molecular Geometry"
AP Classroom: Unit 2 "Molecular and Ionic Compound Structure and Properties" (continued)
AP Princeton Review: Unit 2 (continued)
Topics
- Sigma and Pi bonds
- Hybrid orbitals and Resonance
- VSEPR model of molecular geometry
Labs
- ChemisTREE Silver Ornament lab
- Halogen Compounds lab
- Molecular Shapes weblab
Oil Spill Cleanup research assignment
Instructions below
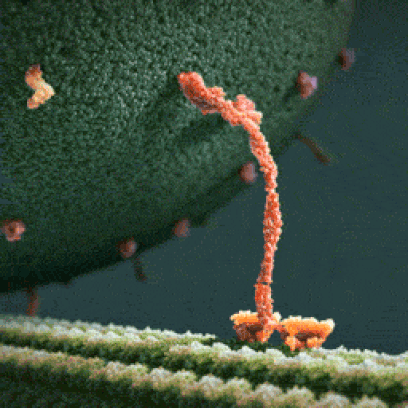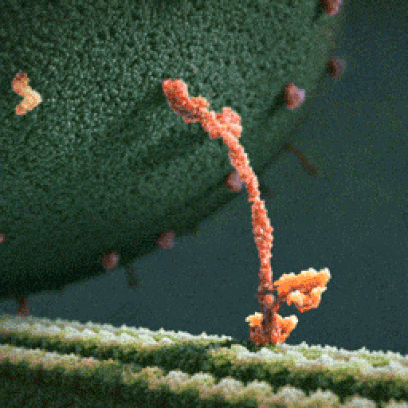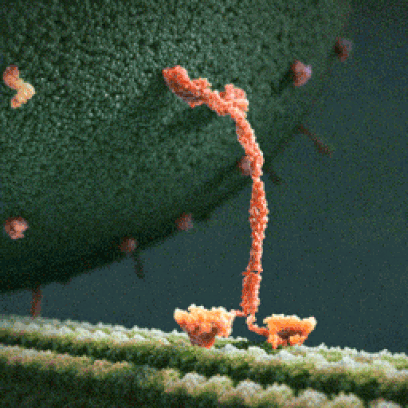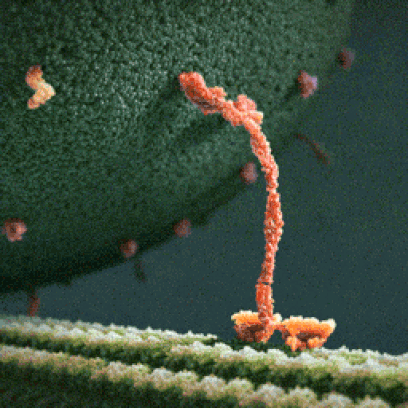
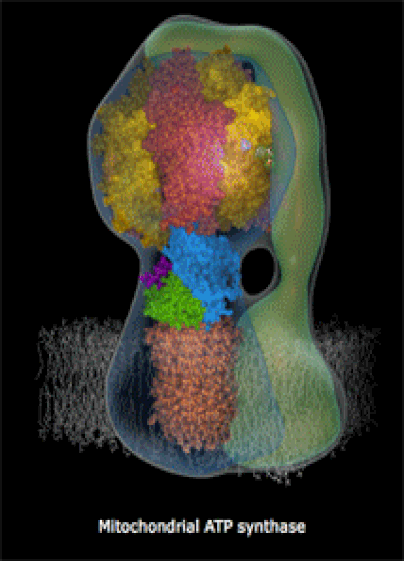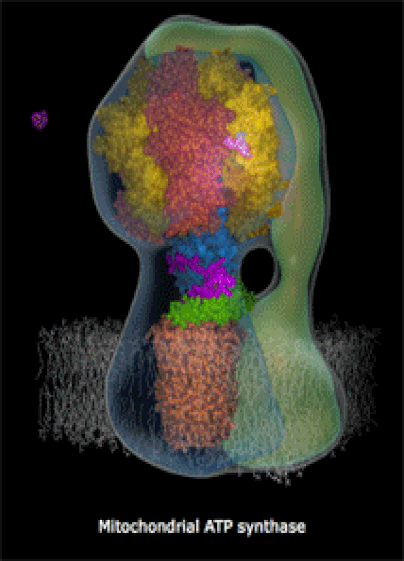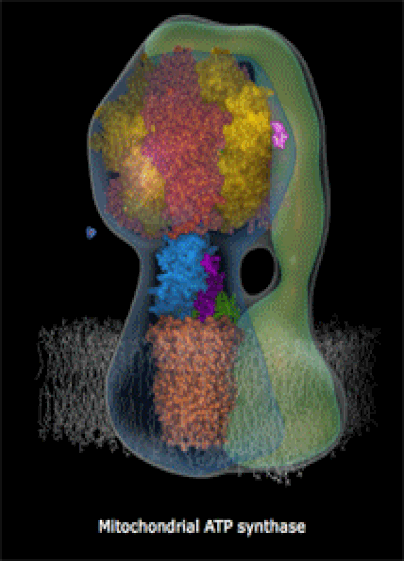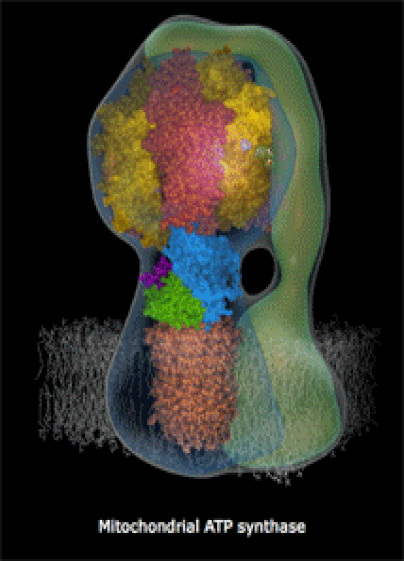
Below: The shapes of large molecules can be quite complex, especially in biochemistry. They can actually be machines!
- Below left: A Motor Protein ("kinesin") transporting a container of protein parts along a microtubule
- Below right: An ATP Synthase molecular turbine charging the ATP "batteries" of the cell
Lecture outline
I. The basis of any bond is a reduction in energy:
I. The basis of any bond is a reduction in energy:
- An ionic bond forms when one atom steals an electron from another atom, and then both atoms are attracted due to having opposite charges. Ionic Bond = outright theft of electrons.
- A covalent bond is when the electron pool is shared. The atoms bond because of attraction of the 2 nuclei for the shared electrons. Covalent Bond = sharing of electrons.
- A polar covalent bond is in-between ionic and covalent. The water molecule is the classic example of a polar bond. Polar Bond = unequal sharing of electrons.
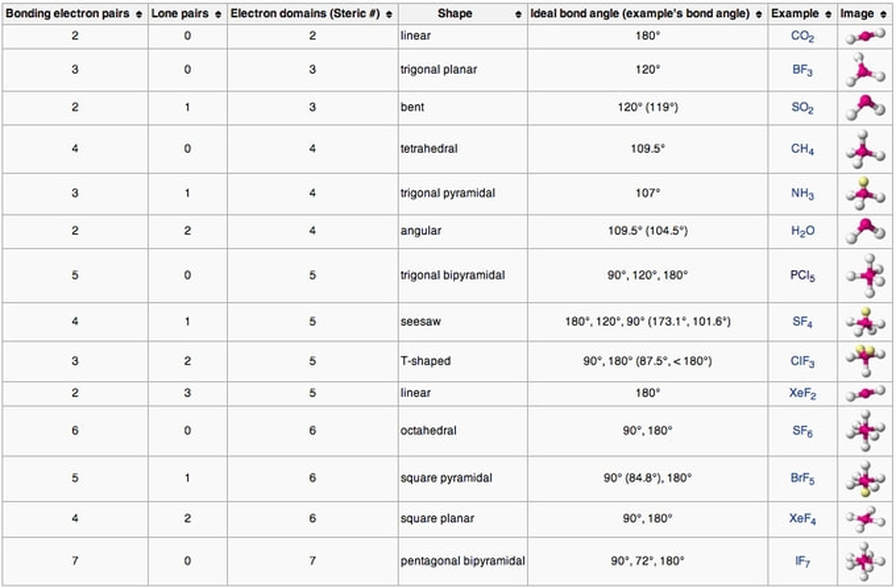
II. VSEPR Model predicts molecule shape:
- The overall shape of the molecule is determined by placing valence electrons (bonded electrons and lone pairs) as far apart as possible. This determines the shape of the molecule.
- Use the VSEPR Chart below to predict bond angles and overall shape:
III. Valence Bond Theory (1916 G.N. Lewis). Also called the Localized Electron Model. The electrons belong to each atom, and occupy the orbitals thereon.
- The tools are Lewis diagrams, the Octet Rule, and hybrid orbitals. Exceptions are allowed for Boron, Sulfur, Nitrogen, and others which don't obey the Octet Rule.
- Resonance (1928 Linus Pauling) is necessary as a band-aid because electrons are not localized on each atom, and everyone knows that. Electrons move around an entire molecule. So we explain multiple-Lewis diagram possibilities for the same molecule by employing "resonance structures".
- VSEPR Model is then used to explain/predict the shape of non-metal molecules. It seems to work, qualitatively at least. The problems that crop up are dealt with as needed.
- Hybrid Orbitals (1930 Linus Pauling) - sp3, sp2, sp, dsp3 - are a modification to account for the observation that certain atoms seem to form special orbitals for bonding, at the expense of native atomic orbitals.
IV. (AP Students) The Molecular Orbital Model (1930's originally called the Hund-Milliken Theory) addresses problems with the Localized Electron Model (the Valence Bond Theory)
- electrons are not localized, and everyone knows that
- the concept of unpaired vs. paired electrons is fishy
- LE model does a lousy job of predicting bond energies
ChemisTREE Silver Ornament lab
In this lab, we will plate Christmas ornaments with real silver, using the famous Tollen's reaction. Everyone will have a silver-plated ornament to take home.
In this lab, we will plate Christmas ornaments with real silver, using the famous Tollen's reaction. Everyone will have a silver-plated ornament to take home.
| chemistree_silver_ornaments_lab_-_student_examples.pdf |
| silver_ornament_plating_lab_handout_2023.docx |
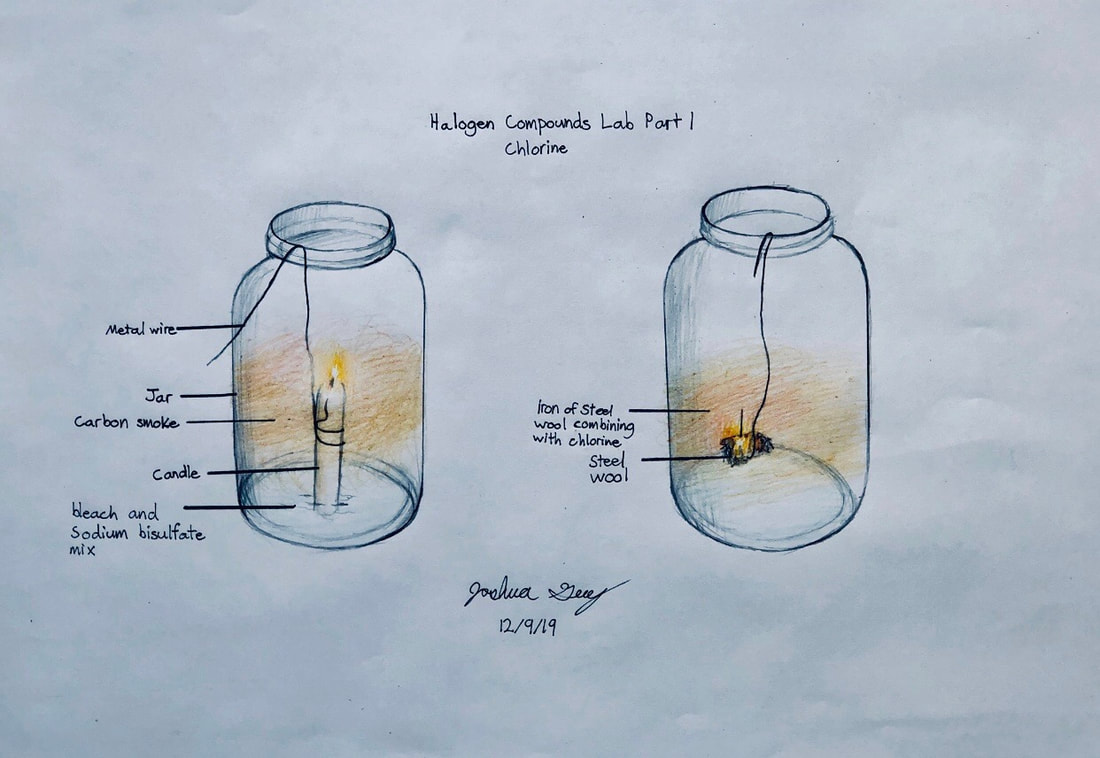
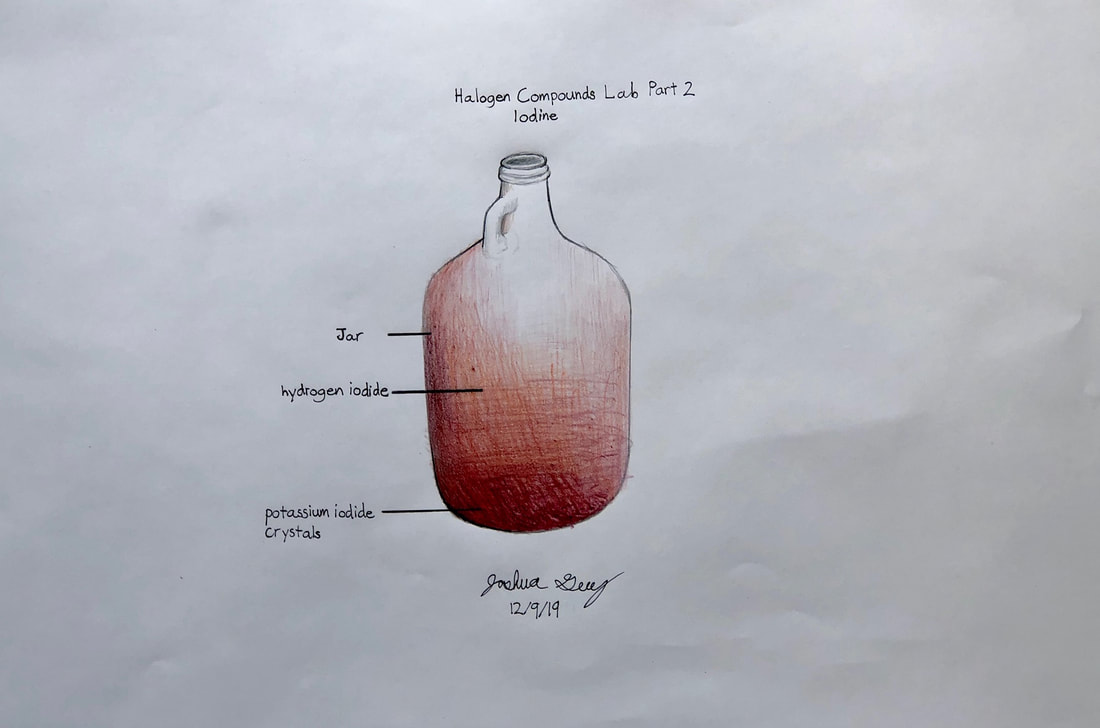
Halogen Compounds lab
This is a classic chemistry lab comparing compounds made from chlorine, iodine, and bromine which we will make and observe.
This is a classic chemistry lab comparing compounds made from chlorine, iodine, and bromine which we will make and observe.
- The halogens are found in Group 7 (or 17) of the periodic table.
- Halogens have 7 valence electrons, so they would prefer to steal or borrow an additional electron from a metal like sodium or calcium, for example.
- The halogens are reactive. Chlorine acts just like oxygen, for example, attacking metals aggressively. You can "burn" pure iron in a container of chlorine gas.
- Fluorine is extremely reactive. It is called the Tiger of Chemistry. We will not be using fluorine in this lab exercise, as it tends to be dangerous.
|
| ||||
Molecular Shapes webLab
| 6._molecular_shapes_weblab_2023_docx |
| h2si2_disilyne_avagadro_screenshot.png |
| n2o4_dinitrogen_tetroxide_avagadro_screenshot.png |
Oil Spill Cleanup research assignment
Research and submit a 1-1/2 to 2 page report on the following – “The Deepwater Horizon explosion in 2010 spilled 5 million barrels of oil in the Gulf of Mexico. How could you use chemistry to clean up an oil spill? Describe what chemicals could be used, the mechanism by which they work, and any equipment which may be needed in the overall process. Include pictures and diagrams which help explain the process.”
Research and submit a 1-1/2 to 2 page report on the following – “The Deepwater Horizon explosion in 2010 spilled 5 million barrels of oil in the Gulf of Mexico. How could you use chemistry to clean up an oil spill? Describe what chemicals could be used, the mechanism by which they work, and any equipment which may be needed in the overall process. Include pictures and diagrams which help explain the process.”
2023 class: You will create a 5-minute teaching video instead of writing a paper. Here is a student example to give you ideas....