Unit 2
The chemistry of life
Reading
Read Ch. 2 "The chemical level of organization"
Read Ch. 2 "The chemical level of organization"
| biomolecules lecture slides.pptx |
Summary
In this Unit, we learn about the 4 chemical groups which make up the body: Proteins, carbohydrates, lipids, and nucleic acids. You obtain these by what you eat (uptake) and by what your body manufactures (synthesis).
In this Unit, we learn about the 4 chemical groups which make up the body: Proteins, carbohydrates, lipids, and nucleic acids. You obtain these by what you eat (uptake) and by what your body manufactures (synthesis).
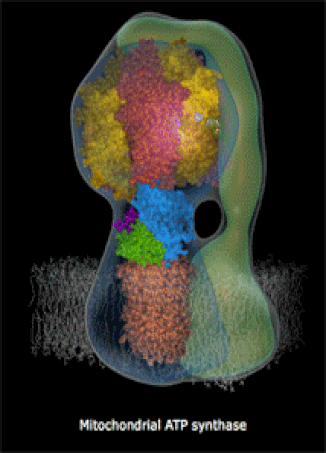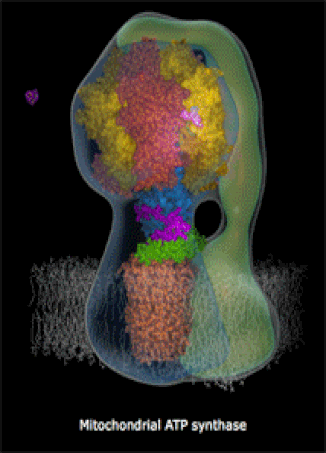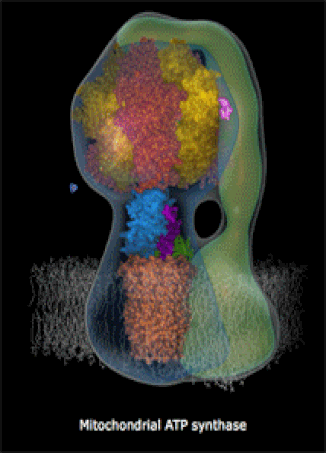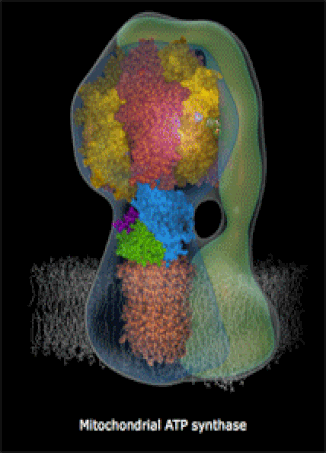
The molecules in your body's cells can be quite complex! The motor protein (left) and the ATP Synthase turbine (right) are nano-machines which your cells build at the molecular-scale (very small machines, way too small to see with a microscope). Every living thing, whether a human, a fruit fly, or a corn plant, has billions of molecular machines.
Video below, 3-min: "Journey Inside the Cell": shows how the big molecules in the cell function
Lecture outline
There are 4 biochemical groups: Proteins, Carbohydrates, Lipids, and Nucleic Acids. We obtain these molecules through what we eat (dietary uptake) and what our cells manufacture (biomolecule synthesis).
Proteins:
Carbohydrates:
There are 4 biochemical groups: Proteins, Carbohydrates, Lipids, and Nucleic Acids. We obtain these molecules through what we eat (dietary uptake) and what our cells manufacture (biomolecule synthesis).
Proteins:
- Proteins are constructed from long chains of amino acids.
- They are the ‘workhorses’ of the cell. They function as the structural parts of the cell, the chemical messengers of the cell, and the numerous enzymes which enable the cell’s metabolism to proceed.
- It is believed that there are around 200,000 different specific proteins in our bodies. Discovering new proteins, and what they do, is a major focus of research in biomedicine and other areas.
Carbohydrates:
- Carbohydrates are made of carbon, hydrogen, and oxygen, always in a C1H2O1 ratio.
- Simple carbohydrates are the sugars such as glucose, sucrose, and fructose.
- Complex carbohydrates include starch, cellulose, and glycogen.
- Carbohydrates perform many functions in the cell. They form the structural elements of plants (cellulose), they function as intermediate energy storage for the body (glycogen) as well as quick energy (glucose), and perform many ‘packaging and shipping’ functions in the cell by serving as chemical markers
Lipids:
Nucleic acids:
- Lipids (fats) are the long term energy storage source in the body.
- Lipids also provide insulation, padding/protection, and lubrication functions
- Lipids also provide the membranes of the cell. Cell membranes are constructed of phospholipids arranged in a double layer, called a “phospholipid bilayer”.
- In the body, fats consist of three long carbon-hydrogen chains bonded at one end to a glycerin molecule, from where they get the name ‘triglycerides’.
- Lipids contain 9 kcals/gram of energy, compared to just 4 kcals/gram for proteins and carbohydrates. This is because they are more ‘reduced’; i.e. there are more C:H chemical bonds which can be oxidized to produce energy
Nucleic acids:
- The nucleic acids, DNA and RNA, contain the coded information necessary for all life.
- DNA is the 'blueprint' or 'parts list'
- RNA is the ‘Post-It Notes’ which carry the instructions out to the cell factory
Enzymes:
- Enzymes are in the protein family of molecules
- They can act as catalysts which enable chemical reactions. An example is amylase, an enzyme in saliva which breaks down carbohydrates into sugars as you chew your food.
- They can also be very complex machines, which perform complicated tasks. An example is ATP Synthase, a rotary turbine in the Mitochondria which spins very fast and charges ATP molecules (we will look at a brief video).
Water:
- Water is the most important and abundant chemical compound in living things
- Almost all the body's chemical reactions occur in a watery medium
- Water is a solvent for ionic or polar substances, for example sugars and salts. (in other words, it keeps these in solution)
- Fats and vegetable oils are hydrophobic, and are not soluble in water
pH scale:
- pH is how we measure acidity
- The pH scale goes from 1 to 14.
- Anything below 7 is acidic; above 7 is basic; and 7.0 is neutral.
Lab handouts
| o2_lab_handout.pdf |
| co2_lab_handout.pdf |
| 2._food_tests_lab_handout_for_starch_sugar_proteins_lipids.docx |
Client Report for Hans Spielman
| client_report_for_hans_spielman__2021_.docx |
Homework - check your class emails for due dates
| 2._inorganic_chemistry_questions_2021__for_anatomy_and_physiology_..docx |