Unit 12: Properties of Solutions
Reading
BJU Chemistry: Ch. 12 "Solutions"
AP Classroom: Unit 6 "Thermodynamics" (we need to move ahead to Thermo to stay on track)
AP Princeton Review: Unit 6
Topics
Labs
Research assignment
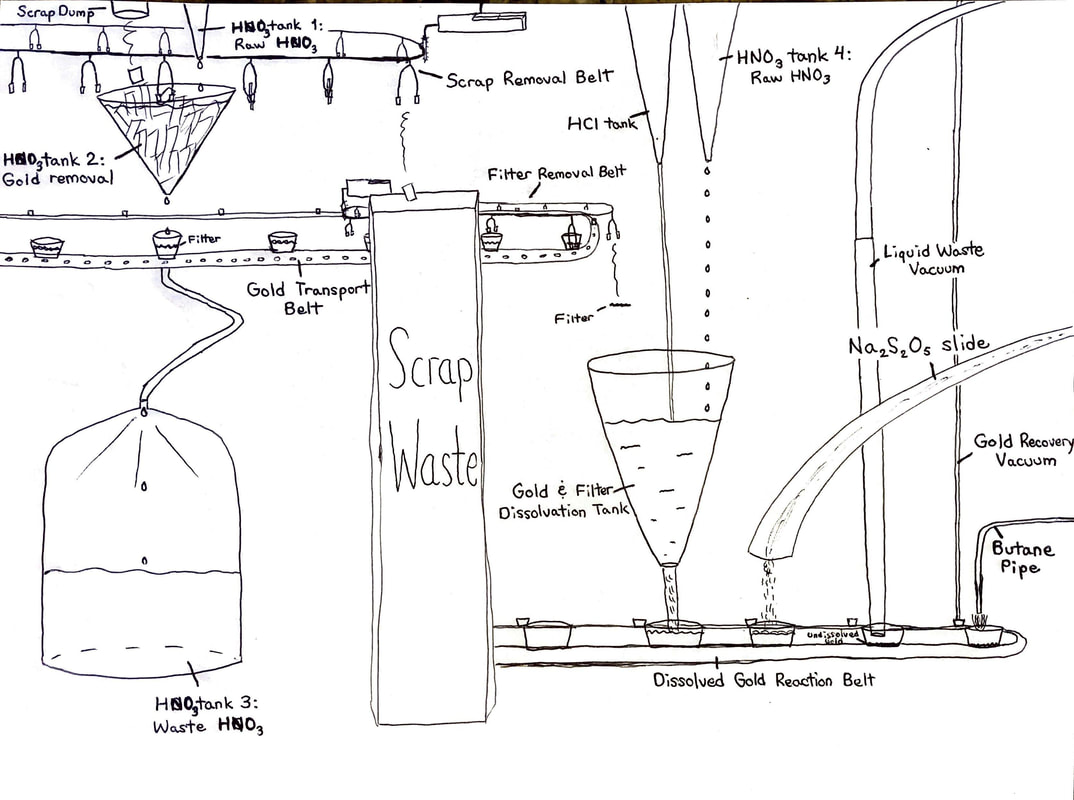
Gold Recovery from Scrap Electronics. Instructions below.
BJU Chemistry: Ch. 12 "Solutions"
AP Classroom: Unit 6 "Thermodynamics" (we need to move ahead to Thermo to stay on track)
AP Princeton Review: Unit 6
Topics
- Types of solutions and their properties
- Solvents and solutes, solubility concepts
- Molarity and percent concentration
Labs
- Environmental Science lab (Treating wastewater using chemistry)
- Distillation of water-ethanol mixture (handout is in the next Unit)
- Concentration weblab (handout below)
Research assignment
Gold Recovery from Scrap Electronics. Instructions below.
Environmental Science lab (treating wastewater using chemistry)
| 12._environmental_science_lab_handout_-_showing_molarity_calcs_5_wastewater_samples_photo.docx |
| 12._environmental_science_lab_handout_-_solns_mixtures_charts.docx |
Concentration weblab
| 12._concentration_weblab__rev_2024_.docx |
Homework
Handouts below. Other homework on Canvas.
Handouts below. Other homework on Canvas.
| 10._know_your_ions_part_2..docx |
Gold Recovery assignment (optional)
| gold_recover_assignment_rev_2021.docx |